A research team led by Dr Andrei Khlobystov of The University of Nottingham’s School of Chemistry has showed that nano-level chemical reactions, which modify the carbon nanotube structures, can be triggered by an ‘attack’ from inside.
According to the findings reported in the Nature Chemistry journal, the carbon nanotubes with modified structures are enchanting new materials that can be utilized in the new technology developments for chemical sensors, gas storage equipment and electronic device components such as transistors.
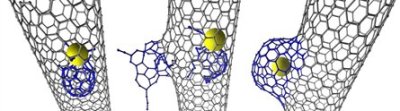
Khlobystov stated that the new research revealed that when the carbon nanotube cavity has catalytically active transition metals it can participate in surprising chemical reactions. During the study, the researchers observed that an individual Rhenium metal atom triggered a chemical reaction that led to the modification of the nanotube’s inner wall. Initially, a small defect is created by the Rhenium atom in the nanotube, which is then slowly developed into a nano-scale protrusion by consuming more carbon atoms.
The protrusion then quickly develops in size to create a novel carbon structure called a nanobud. Earlier, these nanobuds were assumed to be created on the exterior of the nanotube due to the reactions with carbon molecules on the outer surface such as fullerenes.
The research team in partnership with Ulm University’s Electron Microscopy of Materials Science group was able to capture the images of the chemical reaction between the nanotube and the transition metal atom at the atomic level utilizing the aberration-corrected high resolution transmission electron microscopy (AC-HRTEM).
Source: http://www.nottingham.ac.uk


No comments:
Post a Comment